Q1:
Using your textbook and the class wall charts, identify four elements
by their spectra.
The actual spectra depend on the source of the element, or where
it came from. The standard wall-chart pictures of spectra
are derived from controlled conditions.
Q2:
Sketch each of the four spectra. How do the actual spectra
vary from the standard pictures?
-
- The optical apparatus defracts white light and separates
out the different wavelengths, or colors, of light.
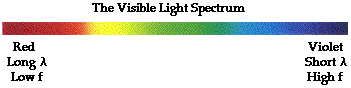 -
- Notice that visible light is only a small part of all the
different types of electromagnetic radiation.
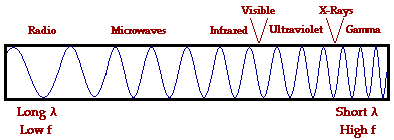
All of these pictures are from "The Physics Classroom"
http://www.glenbrook.k12.il.us/gbssci/phys/Class/BBoard.html
- Q3: How
are these images of spectral lines produced by your optical apparatus?
-
- Spectral lines are a particular set of wavelengths.
Every different type of atom has its own distinctive set of spectral
lines. The lines represent different wavelengths of
electromagnetic radiation, many of which fall into the range of
visible light. The wavelengths of energy are created,
or given off, as electrons in the atom move from higher energy
levels to lower energy levels. An input of energy
is what caused the electrons to move to the higher energy levels
in the first place. Since a particular type of atom
has a certain arrangement of electrons the wavelengths that are
emitted by those electrons are distinctive to the atom.
Q4: How
are these spectral lines generated by phenomena within atoms?
- An understanding of atomic spectra is very useful. It
allows us to easily, and accurately, identify atoms, elements,
and even compounds. This holds true even for identifying
the nature of matter that exists billions of miles from Earth.
|