|
Thermodynamic Applets
The Virtual Laboratory (G. Bothun), University of Oregon, Eugene, OR.
http://jersey.uoregon.edu/vlab
from The Virtual Laboratory (G. Bothun) at the University of Oregon, Eugene, OR.
Three different lessons written using three different applets:
1. Thermodynamic Equilibrium: Mixing Gases of Different Temperature
Looks at the time it takes for a system to come into thermodynamic equilibrium and observe the behavior of the diffusion process.
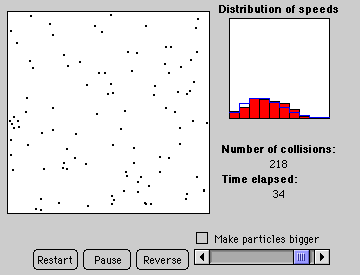
2. Particles in an Atmosphere: Mean Particle Speed and Escape Velocity
Discover the relation: 1/2mv2 = 3/2kT and that P is proportional to T for fixed volume. Note: Excessive pressure in the balloon will cause it to pop (audibly and visually). Also a graphical tool plots the distribution of particle speeds (a Maxwellian) for each temperature.
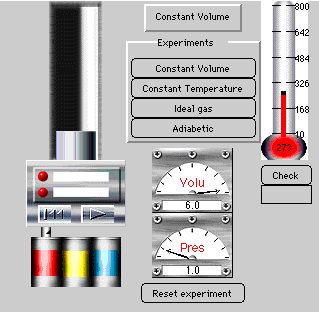
3. Ideal Gas Law: The Piston in the Cylinder
Discover Charles' law by changing the temperature under
conditions of constant volume and observe the change in pressure. The user can hold temperature constant and construct an isotherm as well.
|