| Name _______________________ # ______ |
Date
______ |
Name _______________________ # ______ |
Name _____________________ # ______ |
Atomic Spectral Lines | |
|
| |
1.
Use
the voltage source to illuminate a spectrum tube. Point the spectroscope at the
spectrum tube and move it until you see several sharp lines near the right side
of the spectroscope
2. Draw the bright line spectral
lines made by hydrogen, helium, mercury, neon, and krypton below. Match
these lines up with the numbers you see inside the spectroscope.
(15
points)
|
Hydrogen |
|
4
5
6
7 Helium |
|
4
5
6
7 Mercury |
|
4
5
6
7 Neon |
|
4
5
6
7 Krypton |
| 4 5 6 7 |
Questions
1.
What is the job of a
spectroscope? (3
points)
2.
View the light coming
from outside through the spectroscope. Describe how the light you see from
outside different than what you observed when you viewed the light from the
gases? (5
points)
3.
Use the energy diagram
below to answer the following questions.
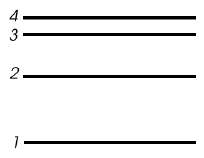
- Draw arrows on the diagram to show all the energy
level transitions an electron could make that would result in the
emission of a photon. (6)
- How many emission lines could we observe from
this atom? ______ (3)
- Which transition would emit light with the
highest frequency (shortest wavelength)?
From n = _______ to n = ________ . (3) - Which transition would emit light with the lowest
frequency (longest wavelength)?
From n = _______ to n = ________ . (3)